Roll-out of second Ebola vaccine confirmed amid criticism
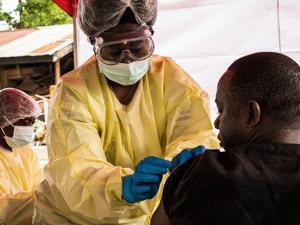
The World Health Organization (WHO) has confirmed the Democratic Republic of the Congo (DRC) will begin using a second Ebola vaccine as part of efforts to curtail the epidemic that has killed more than 2,000 people in the last 13 months.
The experimental vaccine, manufactured by the US-based firm Johnson & Johnson (J&J), will be introduced from mid-October in areas which do not have "active Ebola transmission", the United Nations' health agency said in a statement on Monday.
The J&J product will complement another experimental vaccine, produced by US pharmaceutical giant Merck, which has been administered to approximately 225,000 people to date. Neither Merck's nor J&J's Ebola vaccines are licensed.
DRC health officials had said on Saturday they planned to introduce the J&J product to tackle the outbreak, the second-worst in recorded history, but did not confirm at the time when it would be rolled out.
The introduction of a second vaccine has been a source of controversy among health officials in DRC, where Ebola erupted in August 2018 in the country's eastern North Kivu and Ituri provinces.
Former Health Minister Oly Ilunga Kalenga, who resigned after being stripped of oversight of the Ebola response in July, opposed the second vaccine's use. He said it had not been proven effective and could confuse local populations already in parts mistrustful over Ebola, given the difference in how it is dispensed.
J&J's vaccine requires two injections eight weeks apart. The Merck vaccine, estimated to be 97.5 percent effective, requires a single shot.
Ilunga said the differing dosing schedules could erode already fragile trust in response efforts and be difficult to implement in eastern DRC, where there are large numbers of displaced people and much of the population is highly mobile.
In his resignation letter, published on July 22, Ilunga also cited outside pressure to deploy the J&J product and accused unspecified "actors" of showing a "lack of ethics" over the issue.
But several proponents have urged the use of J&J's vaccine, calling for all "available tools and resources" to be deployed to end the Ebola epidemic.
On Monday, health expert Lawrence Gostin said he welcomed the decision to introduce the second vaccine, despite the move being "late", adding it should "widen the scope of the population covered by immunity".
"We need a major increase in vaccination coverage in the DRC ... [so] it must be rapidly implemented," Gostin, faculty director at US-based Georgetown University's O'Neill Institute for National and Global Health Law, told Al Jazeera.
"It is well within the power of DRC health authorities to educate the public without confusing them about the differences in the vaccines. The bottom line is simple; they are both very effective vaccines."
J&J has tested its vaccine on more than 6,000 volunteers in several trials, according to the firm's website.
Gostin's comments came as medical charity Doctors Without Borders (Medecins Sans Frontieres, or MSF) on Monday criticised the pace of ongoing inoculation as "too slow" in a stinging assessment of WHO's response to the Ebola crisis.
MSF singled out the agency for imposing "tight controls on supply and eligibility criteria" over Merck's product and said an international, independent committee should be created to oversee vaccination efforts instead.
WHO's approach is centered on a so-called "ring strategy", whereby all people found to have come into contact with someone with a confirmed case of Ebola are given the vaccine.
On occasions, blanket "targeted geographic vaccination" of entire neighborhoods deemed to be at high risk from the virus is also carried out rather than vaccinating only known contacts and contacts of contacts.
According to MSF, between 450,000 and 600,000 people should have been immunised by now - more than double the actual number.
"Our capacity to carry out real-time assessments and react accordingly is severely undermined by a rigid system which is hard to comprehend," Natalie Roberts, MSF's emergency coordinator, said in a statement.
"Every day we see known contacts of confirmed Ebola patients who have not received their dose despite being eligible for vaccination," Roberts added.
"It's like giving firefighters a bucket of water to put out a fire, but only allowing them to use one cup of water a day."
Commenting on MSF's criticisms, WHO spokesman Tarik Jasarevic said the agency was carefully managing the "finite number of doses available".
"If all doses were sent to the DRC, there would be no reserves available to respond should cases emerge in any of the high-risk neighbouring countries," Jasarevic told Al Jazeera.
He added that an international committee, such as the one called for by MSF, could only be used to manage licensed vaccines.
"When the vaccine is licensed, this would be an appropriate mechanism for managing supply," Jasarevic said.
So far, more than 3,000 people have been infected by Ebola during the current outbreak in DRC.
Earlier this year, a handful of cases were confirmed in neighbouring Uganda after infected patients crossed the border
All those affected either died or were sent back to DRC for specialised treatment.
Fears of a possible regional spread spiked again over the weekend after WHO criticised Tanzania for failing to share detailed information on suspected cases of the virus.
Toxic mixes of deep-rooted insecurity in the region, widespread mistrust over the outbreak and response efforts have significantly hampered the bid to halt the spread of the virus. There have been regular attacks on medical teams trying to curtail the epidemic.
The worst ever outbreak of Ebola killed more than 11,300 people as it surged through West Africa between 2013 and 2016.
WHO was heavily criticized for its sluggish response to the crisis, which it repeatedly declined to declare a global emergency until the virus spread explosively
